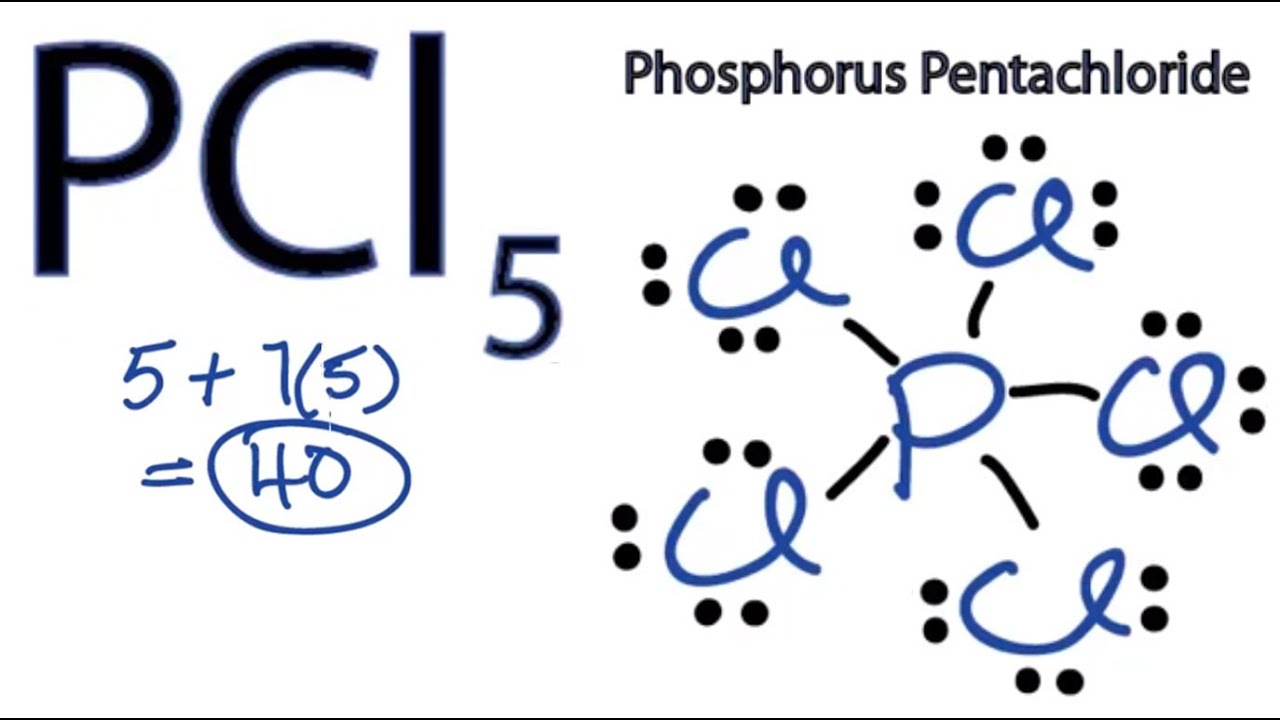
Hence 4 chlorine atoms 3 lone pairs on each 12 lone pairs. Draw the Lewis structure of XeC14.

Pcl5 Lewis Structure How To Draw The Lewis Structure For Pcl5 Youtube
Now lets see the proper steps to draw a lewis structure-1.
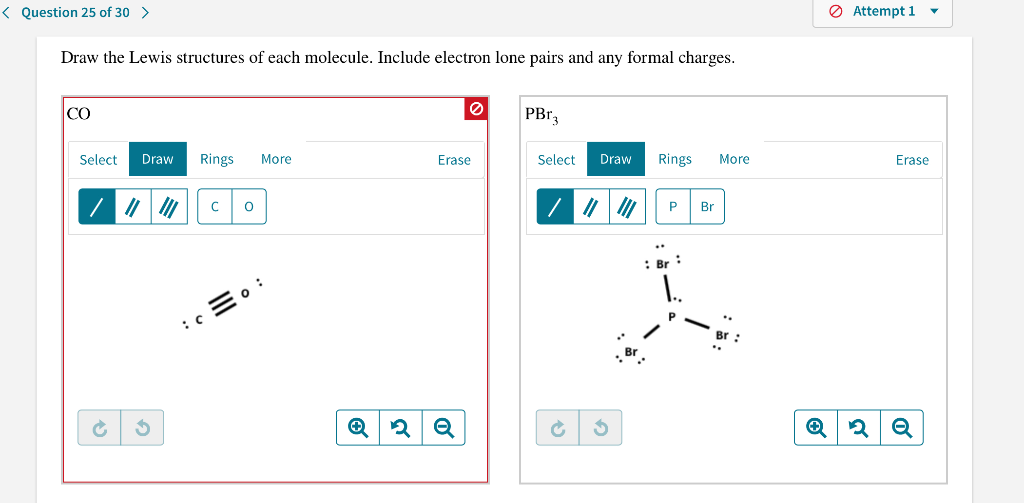
. Draw the Lewis structure of the following molecule. Draw the lewis structure of the following molecule. PBr3 Lewis Structure Molecular Geometry Polarity and Hybridization.
A step-by-step explanation of how to draw the C2H4 Lewis Dot Structure EtheneFor the C2H4 structure use the periodic table to find the total number of val. Include lone pairs NCl3. Find the total number of valence electrons in a molecule- Adding up the valence electrons of all the atoms in a molecule is the first step.
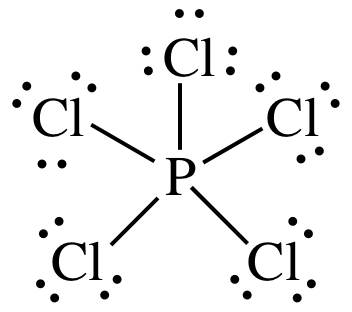
We review their content and use your feedback to keep the quality high. In PCl 5 lewis structure each chlorine atom is joint with center phosphorus atom through a single bond sigma bond. Phosphorus trichloride PCl 3 contains three chlorine atoms and one phosphorus atoms.
You have to follow several steps to draw the lewis structure of NH 3But because ammonia is a simple molecule these steps are not. We can refer to the periodic table for this. See the answer See the answer done loading.
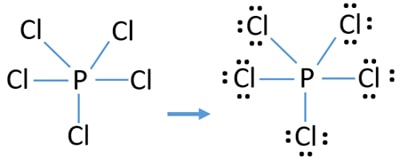
Draw complete Lewis structures including lone pairs for the following compounds. In the SF4 compound there are 4 bonding pairs on the central atom and 1 lone pair of electrons. In this tutorial we will learn how to draw the lewis structure of PCl 5 step by step with all theories.
Steps of drawing lewis structure of NH 3. In PCl 3 lewis structure each chlorine atom is joint with center phosphorus atom through a single bond. Molecular Structure Cont Chemistry terms.
NH 3 lewis structure. In this tutorial we will learn how to draw the lewis structure of PCl 3 with all theories. Also there is a lone pair on phosphorus atom.
One bonded pair contains two electrons hence 4 2 8 bonded pair electrons present in the lewis structure of Silicon tetrachloride. Count the number of valence electrons in a PCl5 molecule. Use symmetry to determine if the molecule is polar or non-polar.
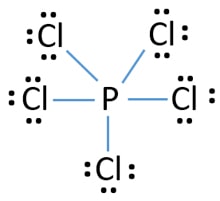
You can see there is no lone pairs on phosphorus atom in PCl 5 as PCl 3. Select Draw Rings More Erase T-shaped Xe Cl trigonal pyramidal square pyramidal tetrahedral see-saw square planar linear bent trigonal planar 2a trigonal bipyramidal OO octahedral What are the. Now the central atom is generally the least electronegative.
PCl 5 lewis structure. For the Lewis structure for PCl5 you should take formal charges into account to find the best Lewis structure for the molecule. Steps of drawing the lewis structure of N 3-ion are explained in detail in this tutorial.
Include all lone pairs. See the answer See the answer See the answer done loading. Draw the Lewis structure Step 2.
H OH a HO b dioxane phenol руmole e f ОНС h HN-C-o. For The Following Molecule And Ion Draw A Correct Lewis Structure. Draw the lewis structure for pcl5 include lone pairs Youll need a gradual hand for this eye catching nail design but it surelys oh-so worthwhile.
There are charges on all nitrogen atoms. Each outside nitrogen atoms have two lone pairs and center nitrogen atom does not have lone pairs. A step-by-step explanation of how to draw the ClF4- Lewis Dot Structure Tetrafluorochlorate ionNote that you should put the ClF4 - Lewis Structure in bra.
This problem has been solved. Identify the molecular geometry of XeCl4. PCl 3 Phosphorus Trichloride Lewis Structure.
This means that it can hold more than 8 valence electrons. In the lewis structure of NH 3 there are three N-H bonds and one lone pair on nitrogen atomThere are no lone pairs on hydrogen atoms which cannot keep more than two electrons. PBr3 is a colorless fuming chemical compound with a strong pungent odor and exists in a liquid state.
The electron density is concentrated along the bond axis. It exhibits a unique property of acting both as a Lewis acid. Also lone pair electrons are also called unshared electrons silicon atoms have no lone pair while each chlorine atom contains 3 lone pairs on it.
If you do not have a gradual hand however there are lots of nail stickers stamps or head on to your neighborhood nail salon so they can replicate it for you. Include lone pairs NCl3. Experts are tested by Chegg as specialists in their subject area.
We come to understand that PCl5 is made up of Phosphorous and Chlorine. Draw the 3D molecular structure w VSEPR rules Step 3. Draw the Lewis structure of the following molecule.
Solution for Draw the Lewis structure of HCN. Each Fluorine atom will have 3 lone pairs and 1 bond. Phosphorus having atomic number 15 has an electron composition of 2 8 5.
Choose a central atom and draw a skeletal structure- Sketch a skeletal of the molecule with only single bonds. A step-by-step explanation of how to draw the PCl5 Lewis Dot Structure Phosphorus pentachlorideFor the PCl5 structure use the periodic table to find the t. Let us now move to SF4s hybridization after we have seen its Lewis Structure.
A step-by-step explanation of how to draw the CCl4 Lewis Dot Structure Carbon tetrachloride. Hybridization is a phenomenon that allows us to understand the geometry of the compound. Drawing the Lewis structure of PCl5.
Who are the experts. The Lewis structure for CCl4 is a commonly tested Lewis struc. In lewis structure of N 3-ion contains two NN bonds.
Therefore it has 5 electrons in its outermost shell. These are the electron dot structure which shows the bonding between the atoms of the molecule and lone pairs of electronif present on an atom Resonance structures. These are the structure which shows the delocalizing of electrons in a moleculeWhen the molecule have more than one Lewis structure those structure are known.
A step-by-step explanation of how to draw the PCl4 Lewis Dot StructureFor the PCl4 structure use the periodic table to find the total number of valence el. Remember when you draw the Lewis structure for PCl5 that Phosphorous P is in Period 3 on the Periodic table. There are a total of 40 valence electrons in the PCl5 Lewis structure.
Solved Question 25 Of 30 Attempt 1 Draw The Lewis Chegg Com
Pcl5 Lewis Structure How To Draw The Lewis Structure For Pcl5 Youtube

Pcl5 Phosphorus Pentachloride Lewis Structure
Pcl5 Phosphorus Pentachloride Lewis Structure
Lewis Structure Of Pcl5 Biochemhelp
Chapter 11 Molecular Geometry Polarity Of Molecules And Advanced Bonding Theory

Draw The Lewis Structure For Pcl5 And Answer The Following Questions A Does The Central Atom Violate The Octet Rule B How Many Lone Pairs Of Electrons Are In The Molecule C
0 comments
Post a Comment